Abstract
Background Systemic mastocytosis (SM) is a rare clonal mast cell neoplasm driven by the KIT D816V mutation in ~95% of patients (pts). SM includes AdvSM (aggressive SM, SM with associated hematological neoplasm, and mast cell leukemia) and non-advanced SM (indolent & smoldering SM), which accounts for ~95% of cases. The uncontrolled proliferation and activation of mast cells can cause severe & unpredictable symptoms resulting in debilitation, reduced QOL, and organ infiltration and damage with reduced survival in AdvSM. While WHO criteria differentiate between subtypes of mastocytosis, there remains a lack of understanding of patterns of disease progression between SM subtypes. In this analysis we developed a novel claims-based algorithm to identify SM cases and assess disease progression.
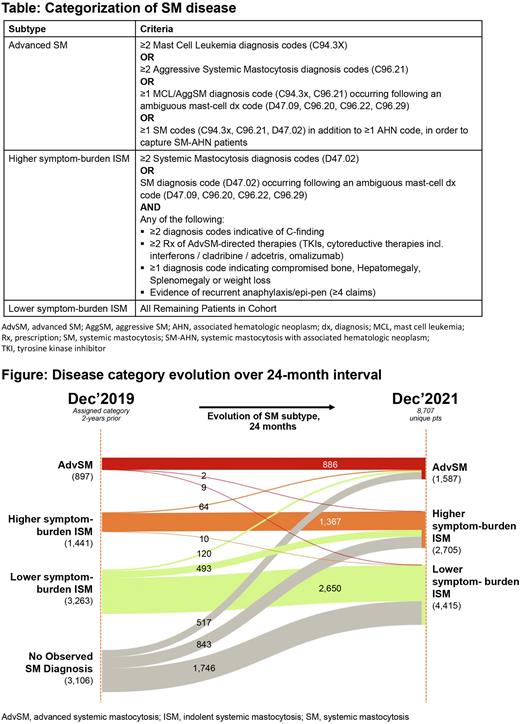
Methods Using a large, nationally representative claims database, we identified a cohort of SM pts with observed claims activity in each of the year 2019-2022. We developed a novel claims-based algorithm based on WHO diagnostic criteria (2016), including codes for laboratory tests, procedures, and treatments typically used for management of SM. Pts were included if they fulfilled 1 of the following criteria: had ≥2 diagnoses with an SM-specific ICD-10 code (D47.02, C96.21, C94.3X) at least 30 days apart in any setting of care; bone marrow biopsy followed by 1 SM diagnosis (dx) claim code in any setting of care; or 1 SM dx code in any setting of care & at least 1 prescription claim for an SM-specific treatment (avapritinib, midostaurin, imatinib, cladribine, interferons, brentuximab, omalizumab, montelukast, or cromolyn sodium). Pts were then categorized as either AdvSM, higher symptom ISM (ISM-higher), or lower symptom ISM (ISM-lower) based on additional disease-specific codes (Table 1) accumulated over the pt's total observable claims history, from 2015 to the date of evaluation. For pts who met these criteria, SM subtyping was evaluated as of Dec 31, 2019, Dec 31, 2020, and Dec 31, 2021. Treatment rates of select SM-directed therapies were also evaluated over the 12- month period from Jan-Dec, 2021.
Results Of 10,939 pts with SM, 80% (8,707 pts) with claims in each of the years 2019, 2020, 2021 and 2022 were included. The first analysis looked at pts’ disease category as of Dec 2021 and assessed utilization of several SM-directed Rx therapies. This included 1,587 AdvSM, 2,705 ISM-higher, and 4,415 ISM-lower pts. Among pts across all cohorts, the most frequently observed therapies were anti-mediator therapies (AdvSM 20%, ISM-higher 37%, ISM-lower 20%) and cromolyn sodium (AdvSM 10%, ISM-higher 19%, ISM-lower 12%).
When evaluating disease category evolution, 11.3% and 18.8% of pts with ISM-low migrated to ISM-higher or AdvSM over a 12-month (Dec 2020 - Dec 2021), or 24-month (Dec 2019 - Dec 2021) interval respectively. Across all ISM types, 2.2% of pts progressed to AdvSM in a 12-month interval, and 3.9% progressed to AdvSM over a 24-month interval. Among those pts who emerged with AdvSM in a 12-month interval ending Dec 2021 (N=392), 33% progressed from ISM-Lower or ISM-higher while 67% appeared as de novo diagnoses.
Conclusions This study used a large claims dataset to identify pts with SM, classified them with a novel algorithm, and describe patterns of disease progression over a 12-month or 24-month interval. Of note, while this algorithm has been developed with expert clinical input, inherent limitations of claims and a single ICD-10 code for SM leave some ambiguity around the true clinical dx of these pts. Despite this, these analyses reflect the accumulation of severe symptoms over time by a meaningful subset of pts. Approximately 35% of pts with an SM dx did not have a SM dx 2 years prior, possibly due in part to the recency of the SM ICD-10 codes and also raising concerns regarding timely dx. Further, not all ISM pts demonstrated truly indolent disease behavior, illustrated by the 30% of ISM pts categorized as ISM-high requiring greater use of symptom-directed and disease-specific therapies. Finally, the proportion of AdvSM pts on FDA-approved TKI-directed therapies remain low, highlighting large unmet treatment need.
Disclosures
Mukherjee:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant, Research Funding; Blueprint Medicines: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Genentech: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Celgene/Acceleron: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant, Research Funding; Aplastic Anemia and MDS International Foundation: Honoraria; McGraw Hill Hematology Oncology Board Review: Honoraria, Other: Advisor or review panel participant; Partnership for Health Analytic Research, LLC: Honoraria; BioPharm: Consultancy; Jazz Pharmaceuticals: Other: Principal investigator for Investigator Initiated Trials (the Institution gets the funding), Research Funding; Eusa Pharma: Consultancy, Other: Advisor or review panel participant; Teaching and Speaking. Cattie:Blueprint Medicines: Current Employment, Current equity holder in publicly-traded company. Pemmaraju:stemline: Consultancy; abbvie: Consultancy; immunogen: Consultancy; mustangbio: Research Funding; incyte: Consultancy; novartis: Research Funding; pacylex: Consultancy, Research Funding; samus: Research Funding; daiichi sankyo: Research Funding; cellectis: Research Funding; cellularity: Research Funding. Green:Blueprint Medicines: Current Employment, Current equity holder in publicly-traded company. Shaheen:Blueprint Medicines: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal